MGPack™
Evaluate the impact of test products on the gut environment through human clinical trials
What is MGPack™?
MGPack™ scientifically verifies the effects and functions of test products (products/materials) by
evaluating the impact they have on the gut environment through human clinical trials. We provide full
support from designing the clinical trial to its implementation and analysis.
● Full support from planning to operation of clinical trials based on the gut environment
research know-how
● Integrated gut environment evaluation through cutting-edge technology
● “Metabologenomics®” Data analysis and result interpretation by gut environment research
specialists, as well as proposals towards future commercialization
Service Flow
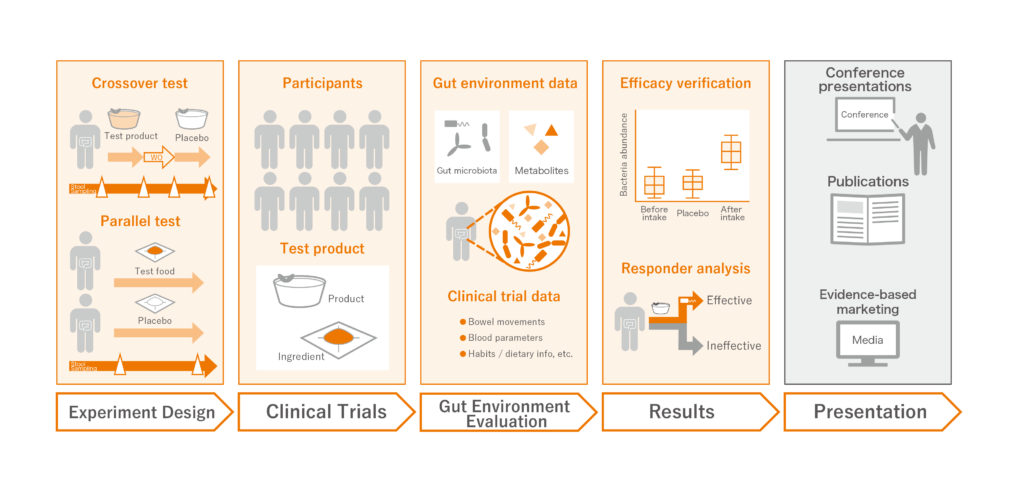
Human Clinical Trials
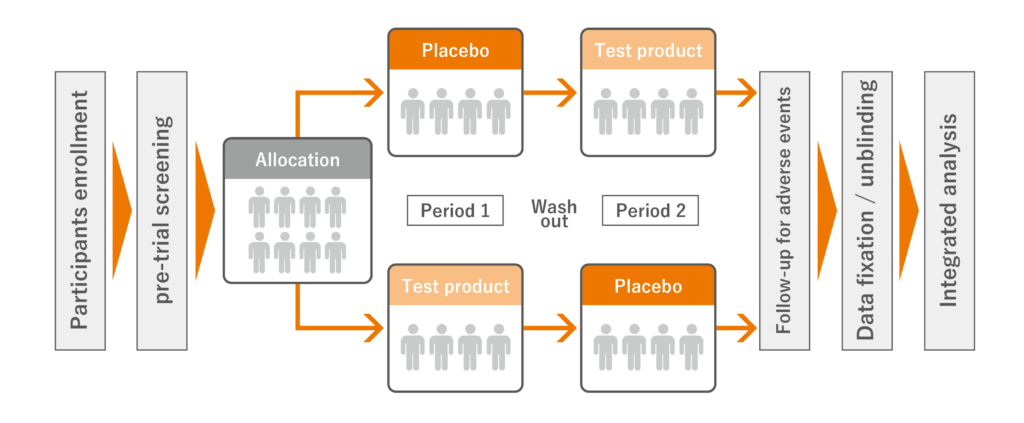
We conduct human clinical trials based on a double-blind, cross-over test (※) on test products (products/materials). To obtain high quality scientific evidence, we utilize the clinical trial know-how that our researchers have cultivated over many years of experience. The basic measurement and analysis items are as follows:
- Metagenome (16S rRNA gene) data of gut microbiota
- Metabolome (metabolite) data
- Metadata such as blood tests, bowel movement frequency, and dietary habits
※A double-blind crossover study is a method in which subjects are divided into two groups and take “test product” and “placebo” at different times from each other without informing which is the test product, and the results of each are tabulated and evaluated.
Gut environment evaluation
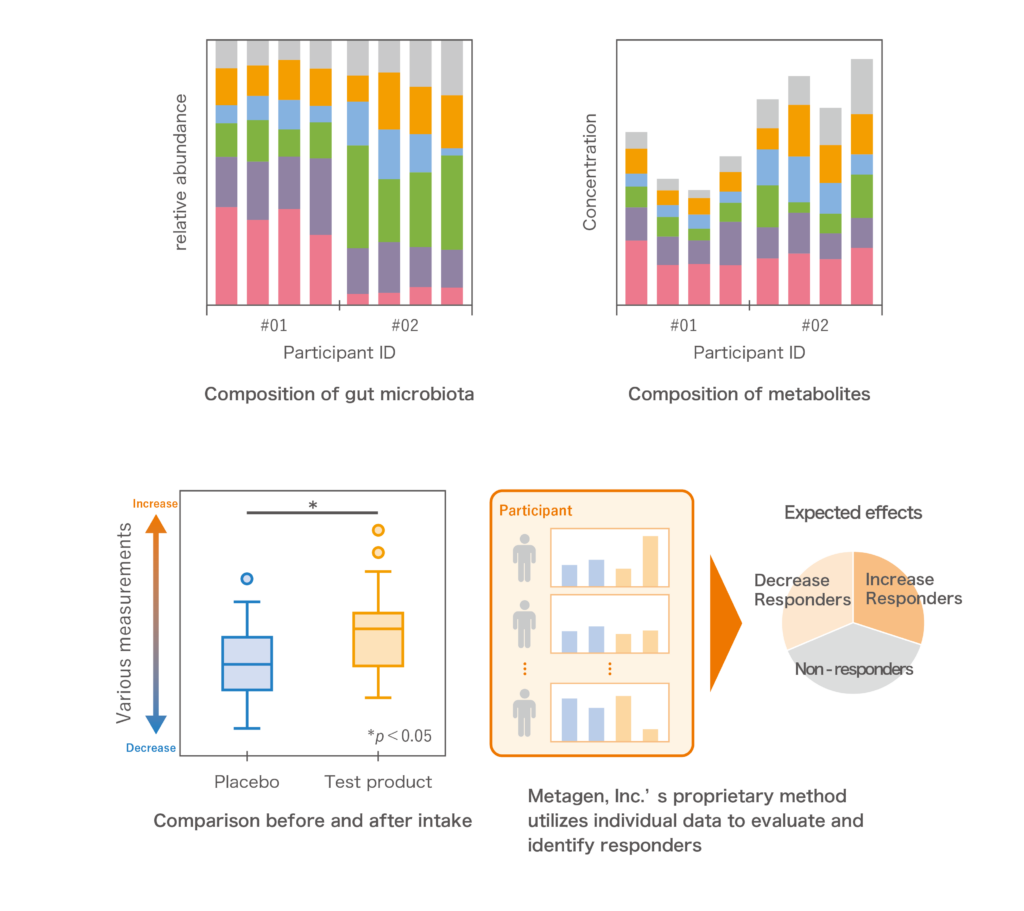
We conduct evaluation on the gut environment using our proprietary Metabologenomics® technology. In conventional gut environment evaluations, bacterial community analysis is commonly performed, but here at Metagen, Inc. we also conduct an integrated analysis of metadata and metabolites produced by gut microbiota. This leads to the elucidation of the mechanism of how gut microbiota work and how it affect the body when test products are ingested.
Research Results
We provide support for the interpretation of results based on the impact test products have on the gut
environment and information about “gut environment characteristics” of people who are more likely
to experience the expected effects. Based on the results, we propose the next step in research and
development and commercialization.
Measurement Items and Options
●Gut bacteria(metagenome) analysis
16S rRNA gene/shotgun
It is also possible to quantitatively measure the amount of a specific gut bacteria using qPCR.
●Gut metabolite (metabolome) measurement and analysis
Determine and analyze short-chain fatty acids (GC-MS) / bile acids (LC-MS) / comprehensive measurement and analysis (CE-TOFMS)
●Responder analysis
People who are more likely to experience the expected effect of test products are so called “responders”. By clarifying the characteristics of responders’ gut environments, you can consider new approaches to expand the potential of test products.
●Immunity Assessment Options
Additional parameters: fecal IgA
●Intestinal Barrier Function Assessment Option
Additional parameters: blood zonulin and LBP
●Conference presentations and paper submissions
As per your request, we will conduct conference presentations and paper submissions together with our company as co-authors. It can be used as evidence-based marketing material or consumer enlightenment.
Example
Verification of effects in bowel movement improvement
- Design: Randomized double-blind placebo-controlled cross-over trial (40 people)
- Number of visits: 5 visits (preliminary examination, intervention I period at week 0 and 4, intervention II period at week 0 and 4)
- Evaluation item: Bowel diary (main evaluation), gut microbiota, metabolites, daily life diary, and other questionnaires (secondary evaluation)
- Analysis items: α-diversity analysis, overview analysis, intergroup analysis, responder analysis, etc.
Expected Results
Analysis results and discussions regarding the mechanisms can be used as new findings and opportunities for developing new products.
Expected Utilization
Utilization for marketing and PR
The research results can be used as reference information for evidence-based marketing and public relations activities at your company. We will also collaborate with media outreach and conducting educational activities, such as holding seminars, if necessary.
※ The labeling of various products and educational activities should be in accordance with the latest contents of the Act against Unjustifiable Premiums and Misleading Representations, the Health Promotion Law, and other relevant guidelines.
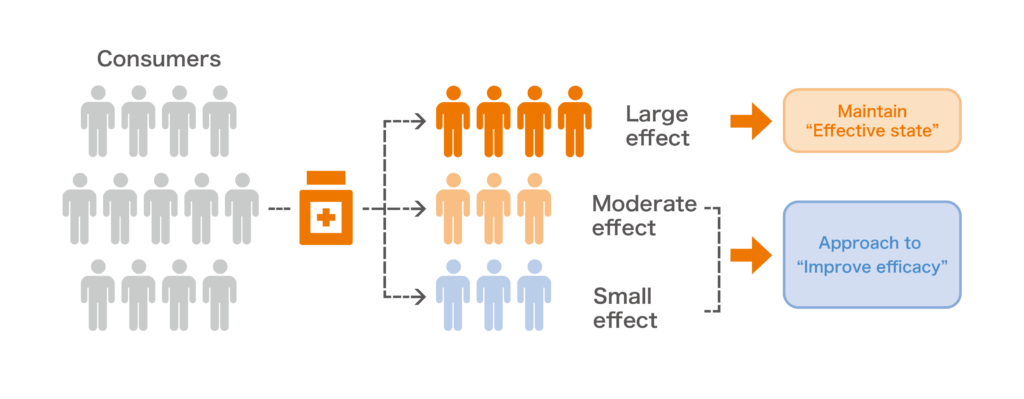
Solution proposal based on the effects of your product
Through the implementation of MGPack™, the “gut environment characteristics” of people who are likely to show the effects due to the ingestion of test products will be scientifically revealed. This will become an opportunity to consider new approaches for expanding the potential of your product. For consumers whose gut environment is more prone to the effects of the company’s products, the study will provide an opportunity for more active promotion, and for consumers whose intestinal environment is less prone to the effects of the materials, the evidence obtained from this study can be used to promote the development of new products such as symbiotics to improve efficacy.
Please feel free to contact us for a quote or to discuss our services or ask any questions you may have.
